In an era dominated by technological advancements and the growing demand for sustainable energy solutions, battery technology plays a pivotal role. Among the various types of batteries, lithium-ion batteries (Li-ion) have emerged as the frontrunners, powering a wide array of devices from smartphones to electric vehicles. But there is growing interest among stakeholders in Nepal and other part of the world on other alternative to this technology. This article aims to provide a comprehensive overview of battery technology, with a specific focus on the evolution of lithium-ion batteries and potential alternatives that could shape the future of energy storage.
The Rise of Lithium-Ion Batteries:
Lithium-ion batteries have become synonymous with portable electronic devices due to their high energy density, longer lifespan, and lighter weight compared to traditional nickel-based batteries. The journey of Li-ion batteries began in the 1970s, but it wasn't until the 1990s that commercial applications started emerging. The development of these batteries owes much to pioneers like John B. Goodenough, Stanley Whittingham, and Akira Yoshino, who were awarded the Nobel Prize in Chemistry in 2019 for their contributions to the development of lithium-ion batteries.
Key Components of Lithium-Ion Batteries
Positive Electrode (Cathode):
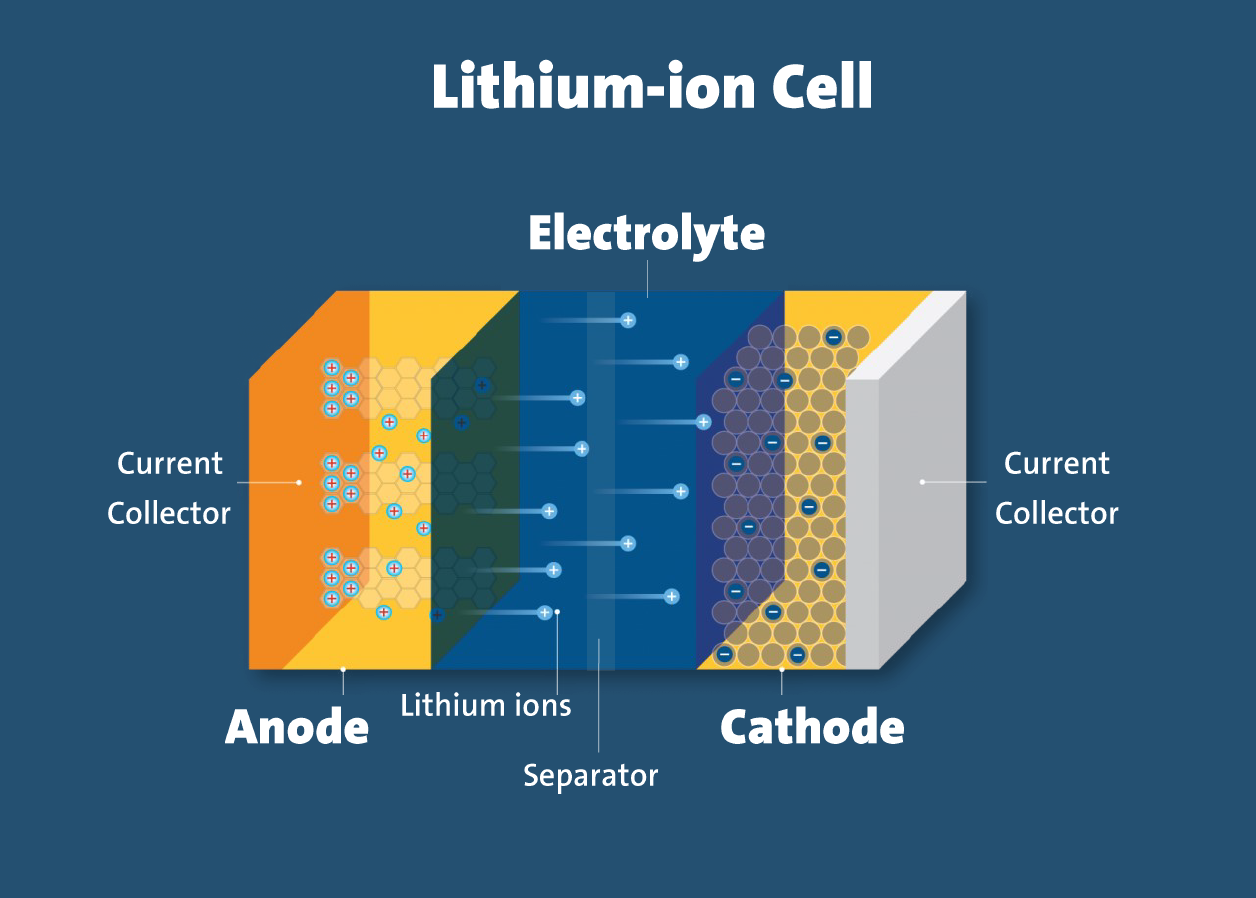
Positive electrode, or cathode is one of the essential players in the battery. It's like the "giver" of positive energy. This part is often made from materials such as lithium cobalt oxide, lithium manganese oxide, or lithium iron phosphate.
Negative Electrode (Anode):
On the other side, we have the negative electrode, or anode. Think of it as the "receiver" of energy. The anode is usually made of a material called graphite, which is a form of carbon.
Electrolyte:
Picture electrolyte as the facilitator, the mediator that allows things to flow smoothly. It's essentially a mixture of a type of salt containing lithium dissolved in a liquid, which acts as a sort of middleman. This electrolyte enables the movement of lithium ions back and forth between the cathode and anode whenever the battery is being charged or discharged.
So, in simpler terms, when you charge your device, the lithium ions move from the positive side (cathode) to the negative side (anode), storing energy. When you use your device, these ions travel back from the negative side to the positive side, releasing the stored energy to power your gadgets.
Evolution and Innovation
In recent years, remarkable progress has been made in improving the performance of lithium-ion batteries through technological innovations. These breakthroughs have resulted in the widespread adoption of lithium-ion batteries, becoming the most prevalent choice for various applications, notably in electric vehicles household electronics and renewable energy storage systems. In Nepal, lithium-ion batteries are go to choice for all EV’s and high precision energy storage use.
Challenges and Limitation
Lithium-ion batteries, despite their widespread usage, are not immune to challenges that necessitate careful consideration.
Limited Raw Materials
One prominent issue is the restricted availability of raw materials vital to their production. Elements like lithium, cobalt, and nickel face constraints due to geopolitical factors, increasing demand, and extraction complexities, posing potential threats to the long-term sustainability of lithium-ion battery technology.
Safety Concerns
Safety concerns constitute another significant challenge, particularly in relation to overheating and fire risks. Although such incidents are relatively infrequent, addressing and mitigating these risks is imperative, especially in applications like electric vehicles where large battery packs are utilized.
Environmental Concern
Environmental considerations also loom large, notably concerning battery disposal. Lithium-ion batteries contain hazardous materials, and improper disposal practices can lead to soil and water contamination.
Future alternative to lithium ion batteries
Sodium Ion Batteries
Sodium-ion batteries, a promising alternative to lithium-ion counterparts, operate by substituting lithium ions with sodium as charge carriers. This single modification holds significant implications for battery production, given sodium's abundant availability compared to lithium. Notably, sodium extraction can be accomplished using salt from oceans, making it a widely accessible resource worldwide. The shift to sodium could potentially reduce the overall cost of battery manufacturing, eliminating concerns related to the storage and transportation of lithium, a potentially hazardous material. Despite these advantages, it's crucial to note that sodium-ion battery technology is in its early developmental stages, and widespread integration into daily life is anticipated to take some time.
Nevertheless, sodium-ion batteries come with their own set of drawbacks. The ions they utilize are larger in size compared to lithium, leading to a reduction in energy density. In practical terms, this could mean diminished electric vehicle ranges and shorter runtimes for smartphones when compared to lithium-ion alternatives. Despite these limitations, the positive aspects of sodium-ion batteries, such as their abundant raw material source, warrant ongoing research and exploration of the technology for potential improvements in performance and applicability.
Lithium Sulphur Batteries
The use of cobalt in the anode of lithium-ion batteries poses challenges due to its difficult sourcing. One potential solution to this issue lies in lithium-sulfur (Li-S) batteries, which employ sulfur as the cathodic material instead of cobalt. Beyond addressing the sourcing difficulties associated with cobalt, Li-S batteries offer notable advantages, including higher energy density and lower production costs, making them an appealing alternative.
However, a significant hurdle for lithium-sulfur batteries lies in their current fast degradation rate. Despite witnessing the application of Li-S batteries in a solar-powered plane as early as 2008, their widespread integration into everyday electronics awaits continued research efforts.
Solid State Batteries
Solid-state batteries utilize a solid electrolyte instead of the liquid electrolyte found in traditional lithium-ion batteries. This design offers several potential advantages, including enhanced safety, higher energy density, and faster charging capabilities. Solid-state batteries have the potential to overcome the safety concerns associated with liquid electrolytes, as they are less prone to issues like overheating and fires. Additionally, the use of solid electrolytes can contribute to longer battery life and increased energy storage capacity, making them a promising candidate for next-generation energy storage solutions.
However, solid-state batteries also face certain drawbacks that need to be addressed for widespread adoption. One significant challenge is the complexity and cost of manufacturing solid-state batteries, which currently makes them more expensive to produce than conventional lithium-ion batteries. Additionally, ensuring the stability and longevity of solid-state battery technology over numerous charge-discharge cycles is an ongoing concern.
In conclusion, despite the drawbacks associated with lithium-ion batteries, their extensive use is unlikely to wane anytime soon. The practicality of transitioning away from lithium-ion technology is less likely especially in countries like Nepal, where the adoption of emerging battery technologies is likely to take years. As advancements continue and research progresses, the energy storage landscape may witness transformative changes, but for now, the reliability and familiarity of lithium-ion batteries will likely maintain their dominance in the foreseeable future.
(The author holds an engineering degree from USA , and is an energy entrepreneur currently working in clean energy and mobility sector in Nepal)